Nanoparticles in the boundary layer of burning iron microparticles – What are the mechanisms behind nanoparticle formation and transport?
New Publication
12.02.2025
Powder for Power: Iron as a Carbon-Free Energy Carrier Metals aren’t just high-energy fuels for rocket propulsion—they can also serve as carbon-free energy carriers! Iron powder offers a unique way to store renewable energy and release it on demand through high-temperature oxidation (combustion). The result? Solid iron oxide (similar to rust), which can be collected and fully recycled—paving the way for a truly sustainable circular economy.
Powder for Power: Iron as a Carbon-Free Energy Carrier
Metals aren’t just high-energy fuels for rocket propulsion—they can also serve as carbon-free energy carriers! Iron powder offers a unique way to store renewable energy and release it on demand through high-temperature oxidation (combustion). The result? Solid iron oxide (similar to rust), which can be collected and fully recycled—paving the way for a truly sustainable circular economy.
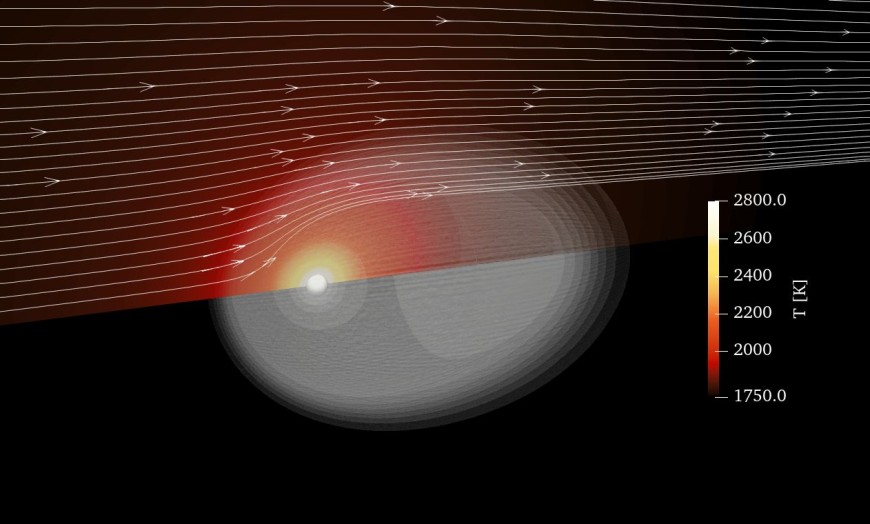
However, a key challenge remains: Nanoparticle formation during combustion. While high temperatures enable fast oxidation and efficient energy release, they also cause iron evaporation followed by gas-phase reactions, leading to the nucleation of ultrafine iron oxide particles.
Our Mission: Understanding the complex interplay of chemistry, heat transfer, and transport phenomena in the boundary layer of burning iron particles. We tackled this challenge with boundary-layer resolved numerical simulations, and our findings have now been published in the Chemical Engineering Journal:
Nanoparticle formation in the boundary layer of burning iron microparticles: Modeling and simulation
By: Bich-Diep Nguyen, Arne Scholtissek, Tao Li, Daoguan Ning, Oliver Stein, Andreas Dreizler, Christian Hasse
Read the full paper here: https://lnkd.in/eA6B-y3b
Key Findings:
- Temperature matters! The iron particle temperature is critical for nanoparticle formation as it dictates iron vapor release.
- Transport mechanisms dominate: Convection and thermophoresis control nanoparticle transport, while diffusiophoresis has little effect.
- Evaporation models are key: The choice of evaporation model significantly impacts the amount of nanoparticles formed.
- Gas-phase reactions don’t trigger nucleation but influence the nanoparticle formation rate.
This study is a step forward in making iron combustion cleaner and more predictable. I am excited to contribute to the future of metal fuels and sustainable energy!
This work was funded by the Hessian Ministry of Higher Education, Research, Science and the Arts – Clean Circles cluster project.

